Alflutop 5 ampoules of 2 ml

2 ml
Convenient for i/a regimen



Alflutop® is used in adults with primary and secondary osteoarthritis of different localization (coxarthrosis, gonarthrosis, arthrosis of small joints), osteochondrosis and spondylosis.


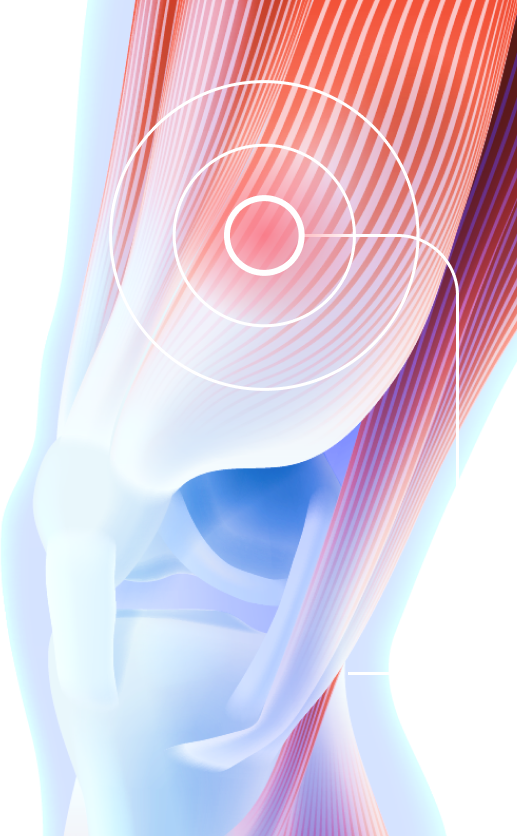
The drug is administered deeply intramuscularly

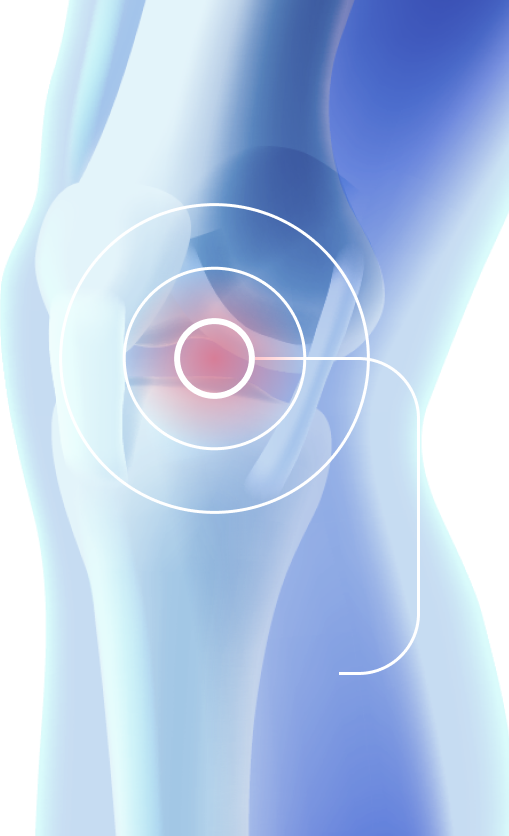
The drug is administered intra-articularly
It is possible to combine
intra-articular and intramuscular
methods of administration
2 courses
per year
Remission
up to 6 months
1 ml or 2 ml in dark glass ampoules with a white break ring. Each ampoule is labeled
5 ampoules in cellular polymer packaging with aluminum foil coating
2 cell packs (1 ml or 2 ml ampoules) or 1 cell pack (2 ml ampoules) together with instructions for use in a cardboard pack

2 ml
Convenient for i/a regimen

1 ml
Convenient for i/m regimen

2 ml
One package per course
Hypersensitivity to the components of the drug. Pregnancy
and breastfeeding period. Children under 18 years of age
(due to the lack of clinical data in this category of patients)
The use of the drug is contraindicated in pregnancy
and during breastfeeding
Side effects are grouped according to the WHO adverse reaction frequency classification: very frequent (≥1/10), frequent (≥1/100 to ≥1/10), infrequent (≥1/1000 to ≥1/100), rare (≥1/10000 to ≥1/1000), very rare (≥1/10000), frequency unknown (frequency cannot be determined on the basis of available data)
Rare: pruritus, skin redness, burning sensation at the site of drug administration, transient myalgias
Very rare: anaphylactic reactions
Frequency unknown: transient worsening of pain syndrome (when administered intra-articularly)
In case of individual intolerance to seafood (marine fish), the risk of allergic reactions increases
Administration of the drug does not affect the ability to perform potentially hazardous activities requiring increased concentration and rapid psychomotor reactions (driving vehicles, working with moving mechanisms, work of dispatcher and operator).
Dose-dependent undesirable drug reactions increase in case of overdose
So far, drug interaction of Alflutop® has not been revealed
Alflutop® is a chondroprotector, the active component of which is a bioactive concentrate from small marine fish. The concentrate contains mucopolysaccharides (chondroitin sulfate), amino acids, peptides, sodium, potassium, calcium, magnesium, iron, copper and zinc ions.
Alflutop® prevents the destruction of macromolecular structures of normal tissues, stimulates repair processes in the interstitial tissue and articular cartilage tissue, which explains its analgesic effect
Anti-inflammatory action and tissue regeneration are based on inhibition of hyaluronidase activity and normalization of hyaluronic acid biosynthesis
Both of these effects are synergistic and cause the activation of regenerative processes in tissues (in particular, the restoration of cartilage structure)
ALFLUTOP®

M09AX (Other drugs for the treatment of diseases of themusculoskeletal system)
П N012210/0
Tissue repair stimulator of natural origin
Solution for injection
The active component of the preparation is a bioactive concentrate from small marine fishes: Sprattus sprattus sprattus sprattus, family Clupeidae; Odontogadus merlangus euxinus, family Gadidae; Alosa tanaica nordmanni, family Clupeidae; Engraulis encrasicholus ponticus, family Engraulidae, obtained by extraction with subsequent deproteinization and delipidization
Active substance:
bioactive concentrate of small marine fishes 0.1 ml
Auxiliary substances:
phenol not more than 0.005 g
water for injection up to 1 ml
Colorless or slightly brownish-yellow or slightly yellow, transparent liquid.
In a place protected from light at temperatures from 15 ° C to 25 ° C. Keep out of reach of children
3 years. Do not use the drug with expired expiration date.
By prescription
K.O. Biotehnos S.A. Gorunului str. no. 3-5, Otopeni town, Ilfov county, 075100, Romania
S.C. ZENTIVA S.A. Teodor Palladi Boulevard No. 50, District 3, Bucharest, 032266, Romania
K.O. Biotehnos S.A. Gorunului str. no. 3-5, Otopeni town, Ilfov county, 075100, Romania
Original complex drug for the treatment of osteoarthritis and back pain


Your question has not been sent. Please try again.
Healthcare Login
All information posted in this section of the website is intended exclusively for healthcare professionals - medical and pharmaceutical workers.
By clicking "I confirm", you confirm that you are a healthcare professional.